QS-21
QS-21 is one of the most successful adjuvants in the current commercial human vaccine market. As a naturally derived saponin mixture, it has been widely applied in the research, development and production of various vaccines. By efficiently enhancing immune responses and inducing long-lasting immune memory, it provides crucial support for the clinical efficacy of vaccines.
Raw Material Source and Production Process
The raw material of QS-21 is derived from Quillaja Saponaria Molina (commonly known as the soapbark tree), an evergreen tree that grows abundantly in Chile. This tree species is rich in saponin resources, and among these saponins, only naturally-sourced QS-21 is used in vaccine production.
Production Process
- Extraction and Isolation: The bark extract of Quillaja Saponaria Molina is subjected to high-purity isolation using chromatography to obtain the QS-21 fraction.
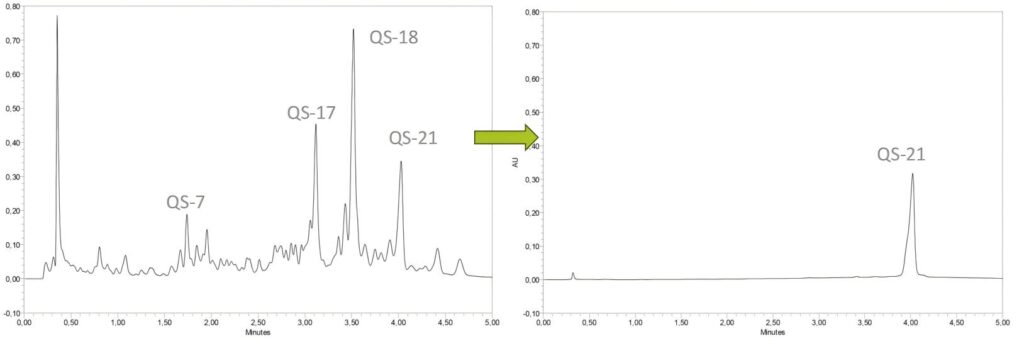
- Manufacturing Facilities: Desert King has established manufacturing facilities in Chile, Mexico, and Italy. Among these, the GMP-certified facility in Italy has been completed, with a current annual production capacity of 5 kilograms. Upon the completion of the expansion project, the capacity will be increased to 10 kilograms (equivalent to 200–400 million human doses).
- Supply Chain Control: Desert King maintains full control over the entire supply chain, spanning from forest-sourced raw materials to the final product, ensuring the stability and reliability of the production process.
Application Scenarios
Approved Commercial Vaccines
QS-21 has currently been validated in 3 commercial vaccines, and is simultaneously used as a core component in various adjuvant systems for a wider range of additional vaccines.| Vaccine Types | Representative Vaccines | Adjuvant System |
|---|---|---|
| Shingles Vaccine | Shingrix | – |
| Respiratory Syncytial Virus Vaccine | Vaccines Related to Arexvy | – |
| Malaria Vaccine | Mosquirix(RTS,S) | ASO 1、ASO 2、ALFQ、Matrix-M |
| COVID-19 Vaccine | COVID-19 Vaccine | Matrix-M |
Clinical Research Directions
Currently, over 20 Phase I-II clinical trials are being conducted for QS-21, covering a range of major disease areas. Notable vaccines under investigation include: HIV vaccines, cancer vaccines, herpes vaccines, Alzheimer’s disease vaccines, Clostridium vaccines, pneumococcal vaccines, hepatitis B vaccines, and anthrax vaccines.
Advantages
- Potent Immune Enhancement: It can simultaneously promote antibody production and cellular immunity, and is particularly effective for vaccines targeting intracellular pathogens (such as viruses and intracellular bacteria) and cancer.
- Significant Synergistic Effect: It can combine with other adjuvants to form composite adjuvant systems (e.g., AS01, Matrix-M), amplifying the immune response effect.
- High Formulation Versatility: It is applicable to multiple formulation types and can support the research, development, and production of vaccines for different diseases.
- Durable Immune Protection: It induces long-term immune memory, providing vaccine recipients with sustained protection for many years.
- Sufficient Clinical Validation: In the shingles vaccine Shingrix, its effectiveness reaches over 95%, fully demonstrating its clinical value.
Supply Security and Sustainability
Supply Capacity
- Desert King International maintains hundreds of grams of current valid GMP-grade QS-21 inventory.
- The Italian GMP facility has an annual production capacity of 5 kg, expandable to 10 kg, supporting 200–400 million human doses .
- Each gram of QS-21 enables 20,000–40,000 human doses . Based on current commercial vaccine consumption (approximately 5 kg/year), this translates to a sustainable supply of billions of doses .
Sustainability Assurance
- Sustainable Raw Materials: Raw materials are harvested via selective bark pruning rather than tree felling. The trees can regenerate in 5–8 years, ensuring circular resource utilization.
- Standardized Supply Chain: It adheres to strict FSC (Forest Stewardship Council) standards and forest rotation systems, protecting natural habitats and biodiversity.
- Sufficient Future Reserves: It owns hundreds of hectares of Quillaja saponaria plantations and has made new progress in cloning technology, which will gradually reduce or eliminate reliance on the harvesting of natural bark.
- Low Resource Proportion: Currently, the content of Quillaja saponin used in vaccines accounts for less than 1% of the total industrial consumption, resulting in minimal pressure on raw material supply.

