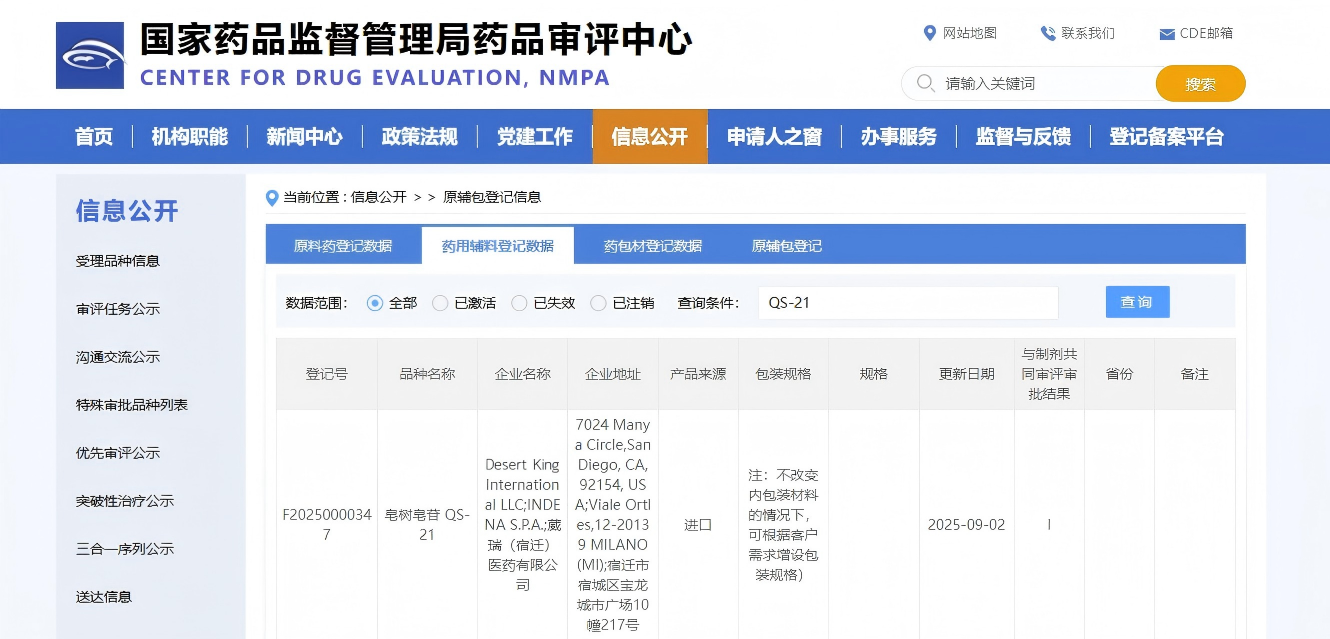
On September 2, 2025, our company received exciting news — the application for the excipient registration of Quillaja saponin QS-21 produced by Desert King International, which we acted as the agent for, has successfully entered the Public Notice Phase I after rigorous review and multiple verifications by the Center for Drug Evaluation of the National Medical Products Administration!
QS-21 is one of the most potent immunopotentiators in the world. It is extracted and purified from the bark of Quillaja saponaria Molina using advanced HPLC separation technology. This special saponin component can induce humoral and cell-mediated immunity and has been used in 5 globally approved vaccines (herpes zoster vaccine, malaria vaccine (Mosquirix; R21), RSV vaccine for adults, and COVID-19 vaccine).
As a leader in the field of pharmaceutical adjuvants, Desert King International, together with VuRoyal Pharmaceutical, has completed the filing and public notice of Quillaja saponin QS-21 on the excipients, packaging materials and adjuvants registration platform of the Center for Drug Evaluation. This achievement is of great significance, marking that this world-leading high-end pharmaceutical excipient has officially entered a new stage of domestic compliant application. As the exclusive agent in China for Desert King International’s vaccine adjuvants, domestic vaccine R&D enterprises and innovative drug R&D enterprises can obtain compliantly supplied high-end excipients through our company.